#LIOS-003
SUMMARY
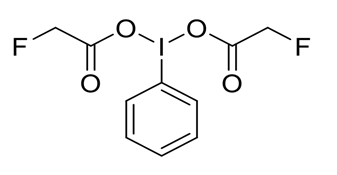
This iodine(III) reagent is of interest to the medicinal chemistry and synthetic organic chemistry communities. It is an efficient and versatile tool for generating new fluorine-containing compounds for drug development. Specifically, photoredox-catalyzed radical fluoromethylation applications of alkenes[1] and heterocycles[2].
BACKGROUND
Organofluorine compounds are of enormous importance in medicinal chemistry, as the fluorine atom can be found in many pharmaceutical drugs. The monofluoromethyl group (–CH2F) is an interesting functional group that could act as an isostere of methyl, thiol, hydroxyl, methoxymethyl, and amine moieties, highlighting its potential value in drug development programs. The current reagent is an efficient and versatile tool for photoredox-catalyzed radical fluoromethylation applications of alkenes[1] and heterocycles[2].
FEATURES AND KEY BENEFITS
- Versatile tool for fluorine-containing compound synthesis (e.g., for new drug development);
- efficient generation of the fluoromethyl radical (CH2F*) under mild visible-light photoredox catalytic conditions;
- a solid, bench-stable reagent;
- ensures radical addition to alkenes or various heterocyclic substrates;
- the most versatile monofluoromethyl radical source with the broadest potential applications in synthesis.
REFERENCES
[1] Ramkumar, N.; Plantus, K.; Ozola, M.; Mishnev, A.; Nikolajeva, V.; Senkovs, M.; Ošeka, M.; Veliks, J. New J. Chem. 2023, 47, 20642-20652. DOI: 10.1039/D3NJ04313D
[2] Ramkumar, N.; Baumane, L.; Zacs, D.; Veliks, J. Angew. Chem. Int. Ed. 2023, 62, e202219027. DOI:10.1002/anie.202219027
AVAILABLE
for licensing or research collaboration.
CONTACT
Dr. Anna Stikāne: anna.stikane@osi.lv
Latvian Institute of Organic Synthesis, Aizkraukles Str. 21, Riga LV-1006, Latvia
CAS: 57357-21-8



